Product Development
Have a new product design or idea? As difficult as it is to conceptualize or design a new product, the real challenge lies in bringing your idea all the way through to market release.
1. Who can help you make prototypes and design the safest product possible?
2. How do you navigate the heavily regulated ophthalmic industry?
3. How do you want to approach the market? Direct sales or distributers?
Peregrine can answer each one of these questions, working with you to determine the best result possible. We work with experienced engineers, employ talented regulatory personnel, and have business relationships with sales representatives as well as the largest suppliers to the market.
All product development is performed to the satisfaction of the USA Code of Federal Regulations Title 21 and ISO 13485, as well as Peregrine Surgical LLC's internal quality standards.
Don't navigate something you may not have experience with. Contact Peregrine Surgical LLC to discuss your options.
Case Study - Adjustable Chandelier Illuminator Development
An existing customer approached Peregrine Surgical LLC to develop and bring to market a new chandelier illuminator.
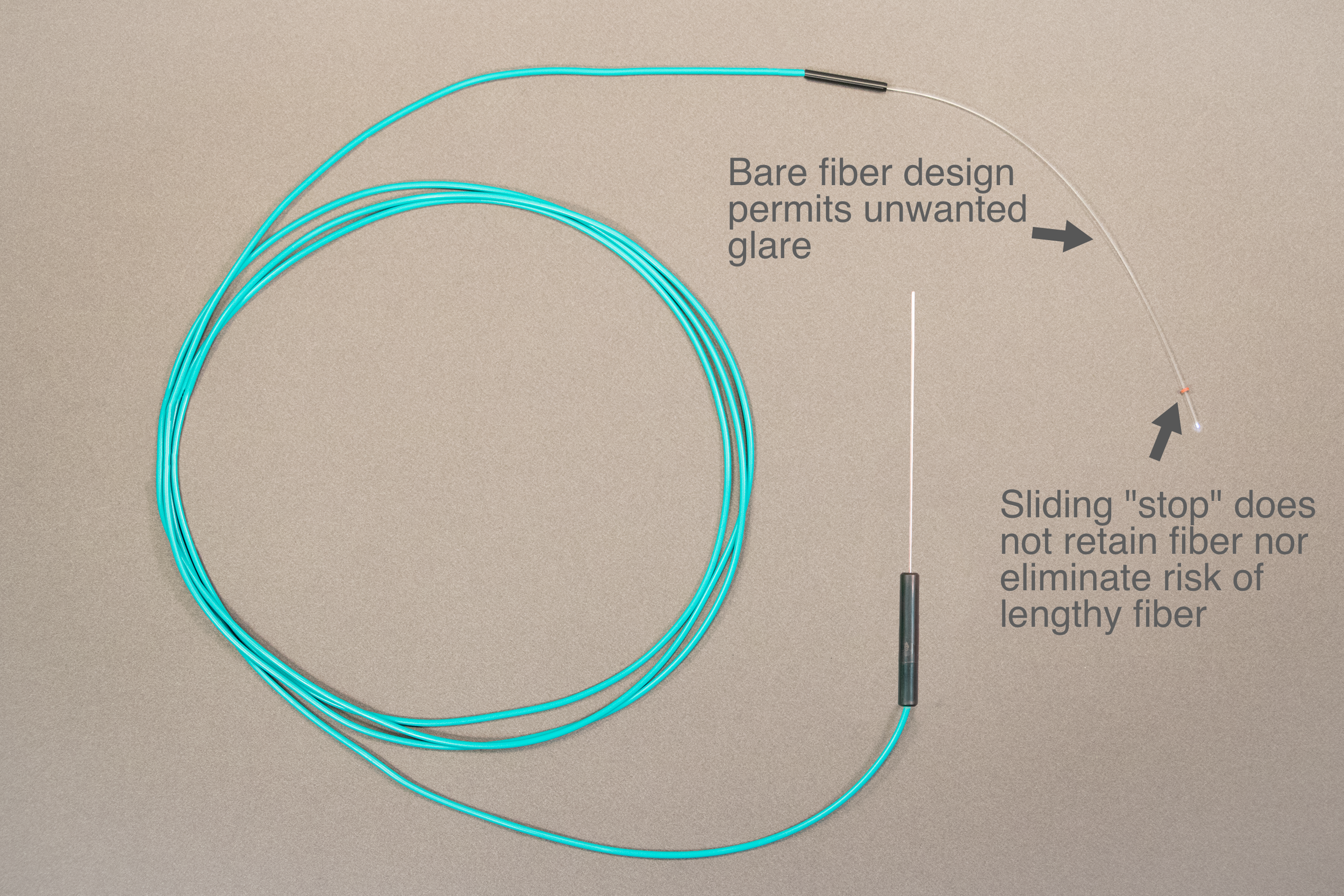
Limitations of Existing Chandelier Illuminators
Bare Fiber - distracting glare from exposed fiber
Risk – sliding silicone “stop” does not prevent possibility of retinal contact by fiber
Inability to secure fiber within cannula
Benefit of hands free device diminished by torque placed on eye from weight of fiber/device
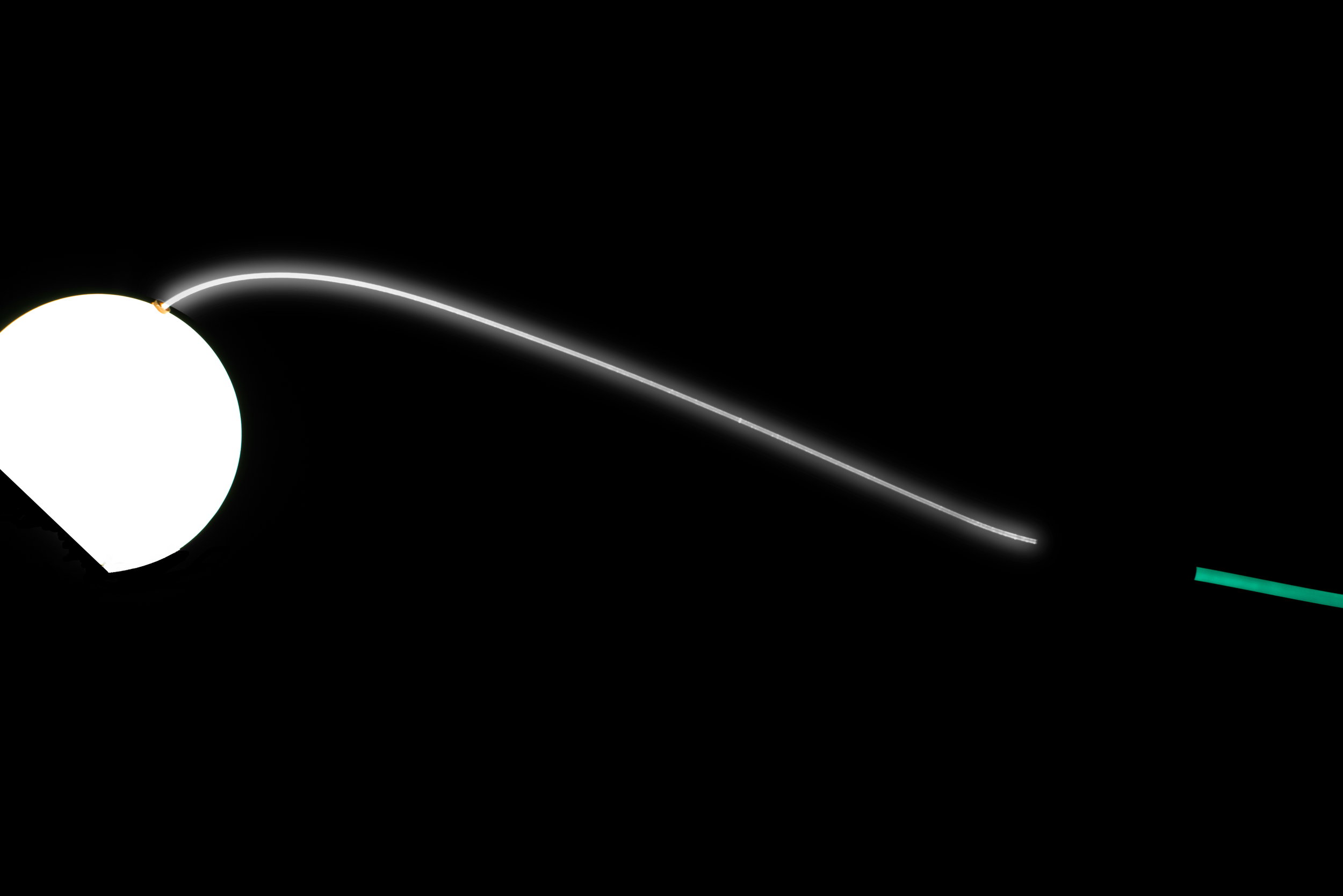
Development Process
Challenges:
Existing Intellectual Property - Existing products on the market required Peregrine Surgical LLC to develop unique and creative approaches to navigate around protected features.
Materials - Desired features of the Adjustable Chandelier Illuminator forced Peregrine Surgical to utilize compatible materials in order to achieve design goals.
Components - Keeping market price in mind, critical components were designed while taking into account function, benefits, and costs.
Validations - Like most developments, initial verifications and validations necessitated revisions. The result is a high quality, unique Adjustable Chandelier Illuminator.
“Each development project is unique, and the Adjustable Chandelier Illuminator was special because of its features and materials. This one took some time, but all of the effort paid off with what I consider an exceptional product.”
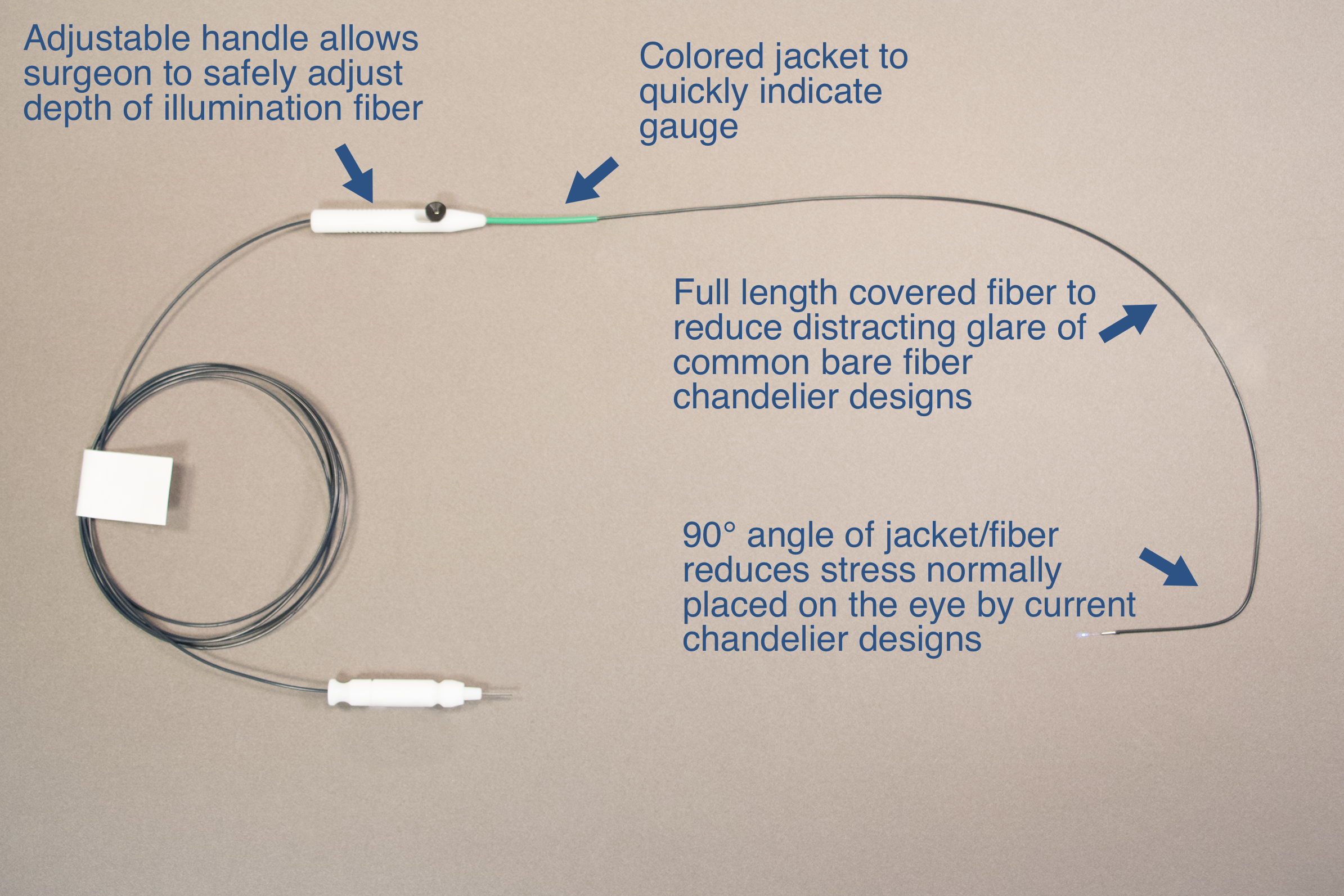
Final Product
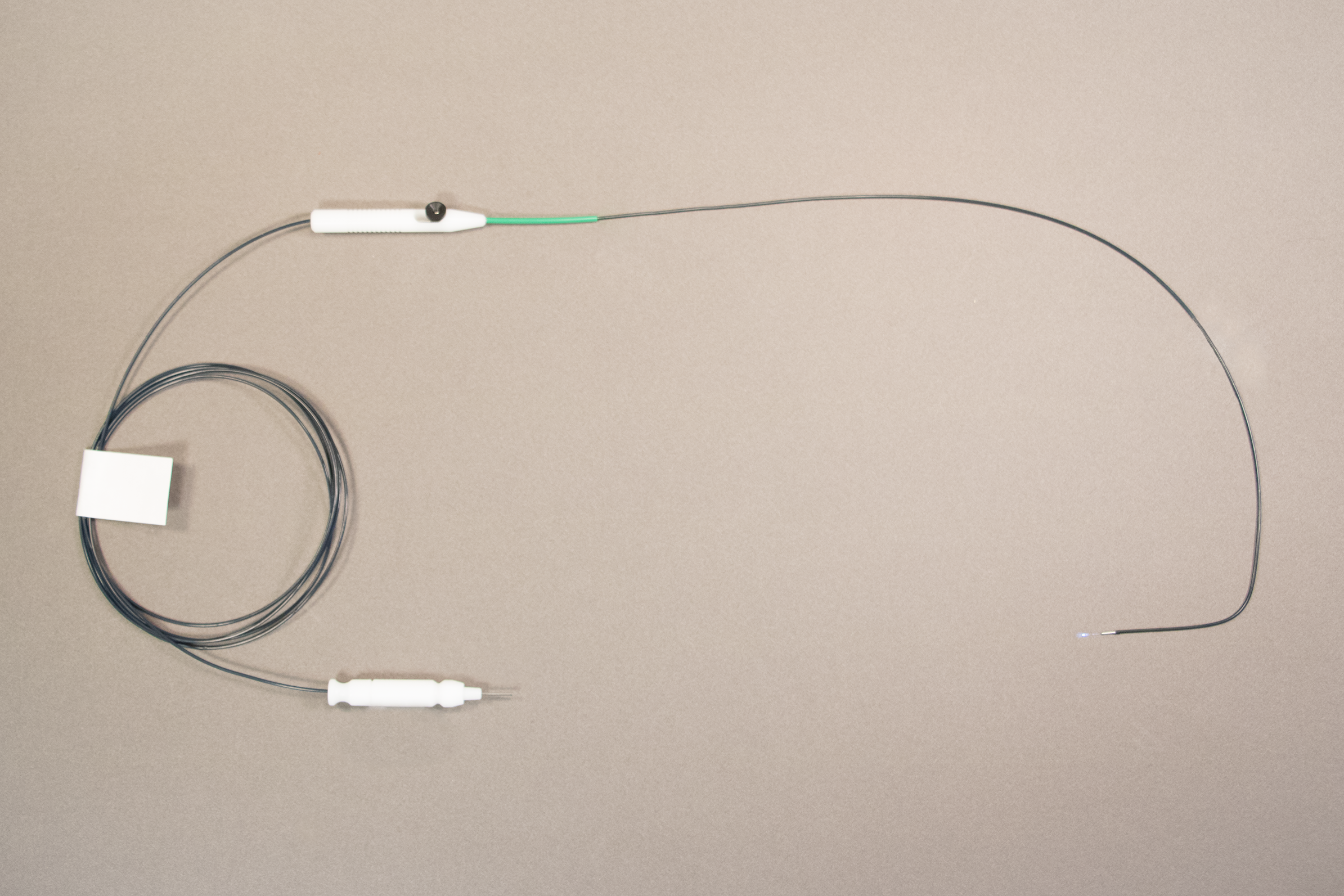

Advantages of New Design
Reduction of glare by introducing a full length sheath/jacket
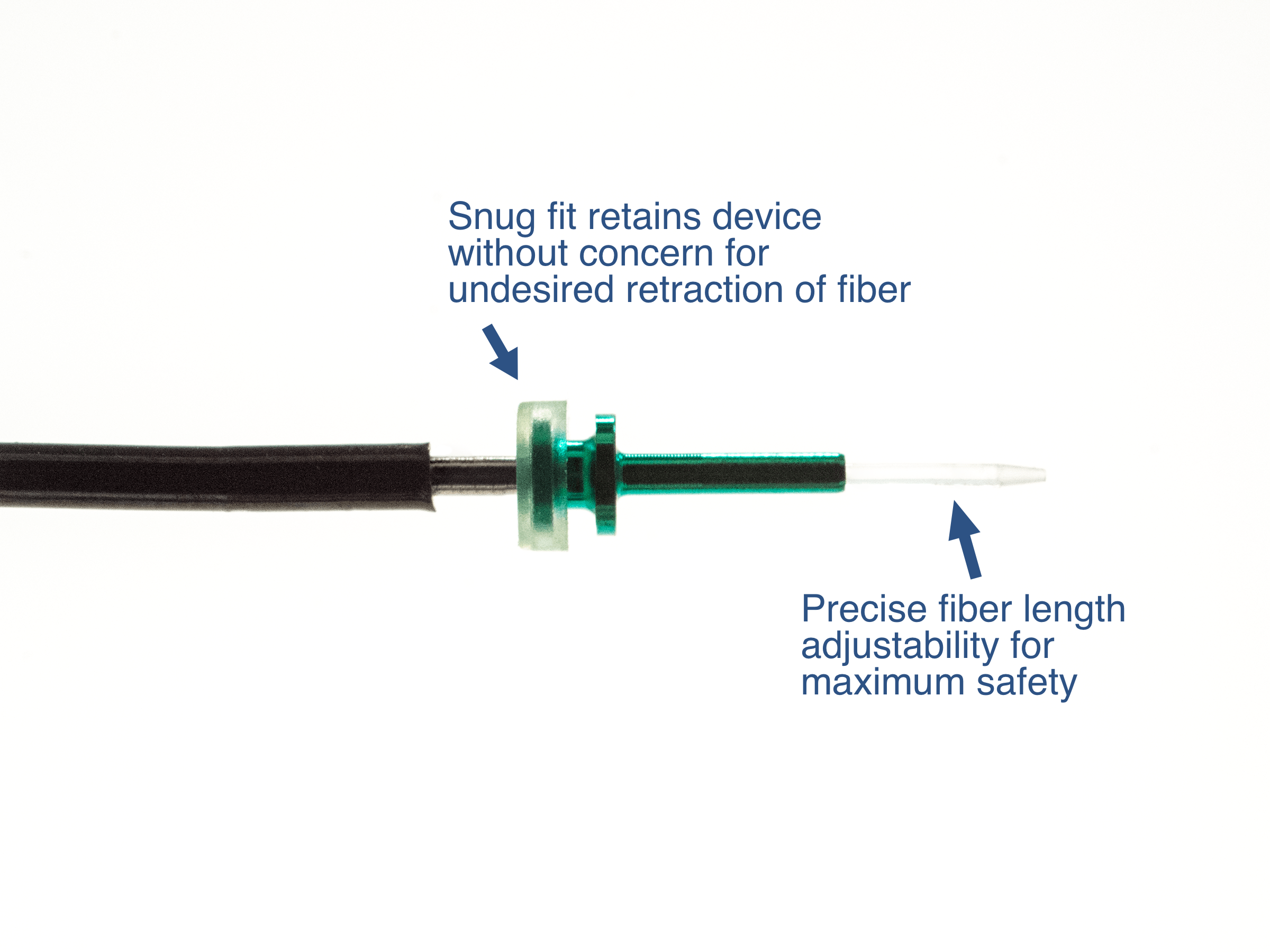
Adjustable Hand piece allows for simple change in fiber position as well as definitive maximum fiber depth
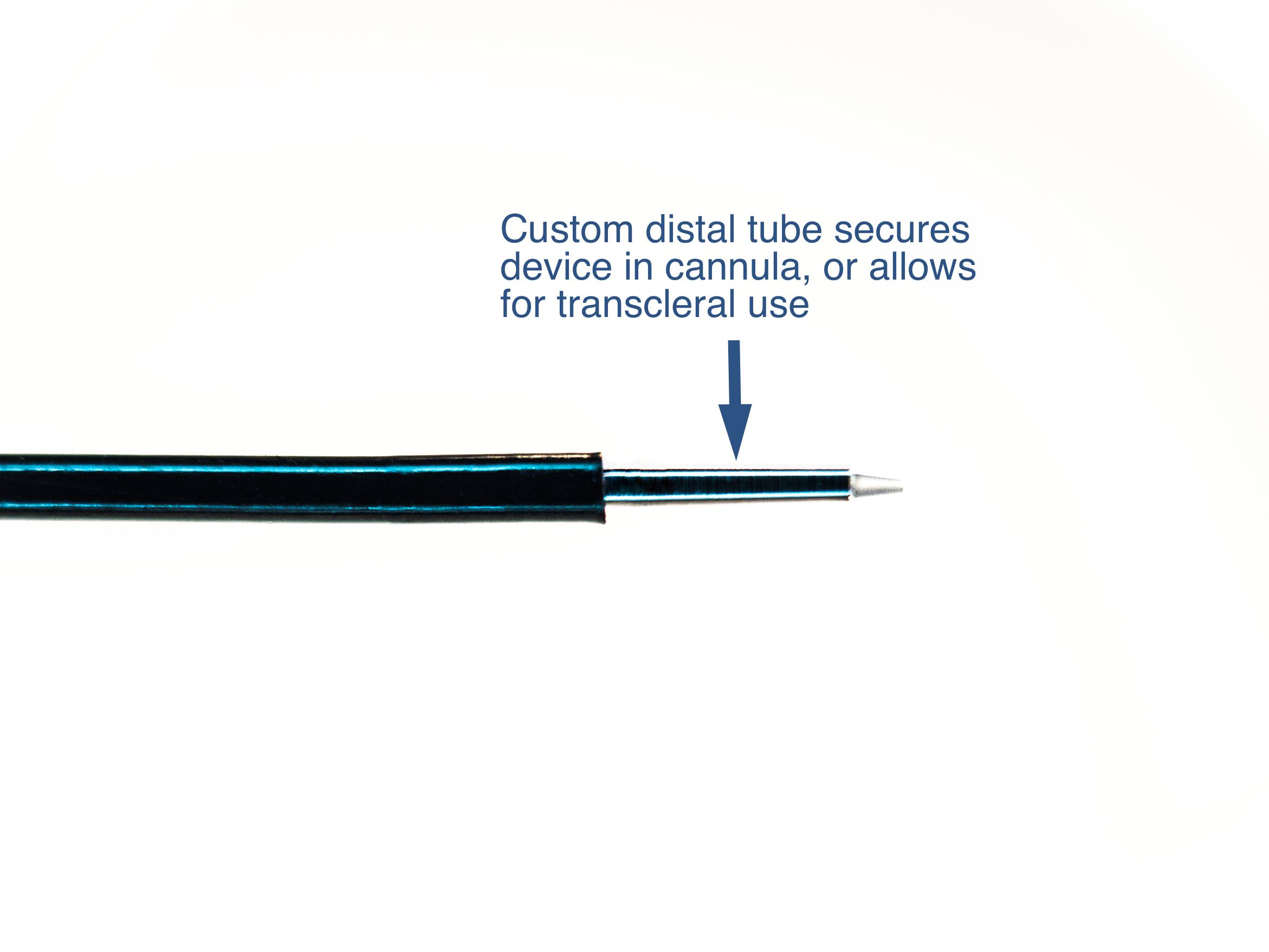
Custom Distal tube to secure device within cannula
Formed fiber/jacket to reduce torque associated with hands free device
Conclusion
The result of this product development is a unique, marketable medical device that takes into account customer requirements, existing intellectual property and user feedback. The Adjustable Chandelier Illuminator is a reliable, patent pending device that better serves the needs of surgeons in terms of safety and functionality.